-
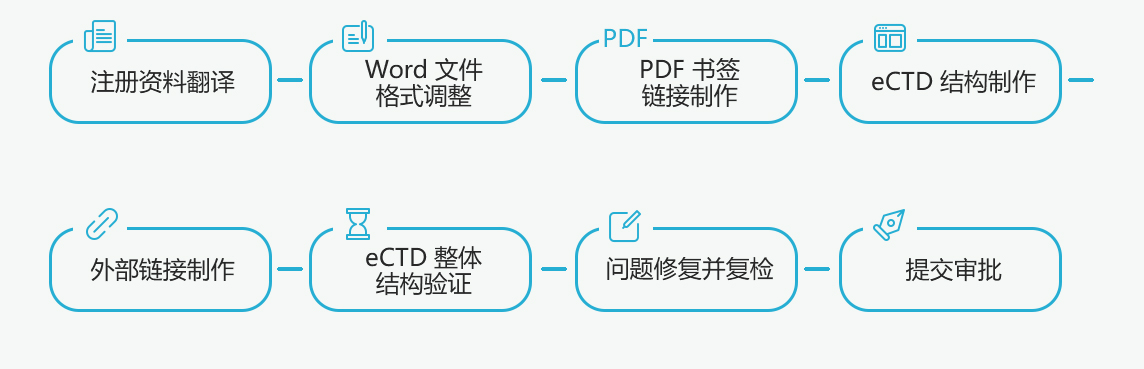
![]() Word file check
Word file check
Check that the page layout, font size and color, text title, table/figure/attachment title and paragraph meet the application requirements;
-
![]() PDF file creation
PDF file creation
Attribute description (including title filling, clear author, PDF version, file size, quick web view), security, font, initial view, corresponding PDF bookmarks, table of contents, links in the document, check the validity of the links one by one, and ensure that There are no omissions and the destination page jumps correctly to ensure the compliance of all PDF files;
-
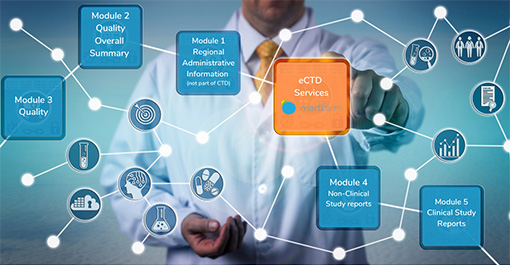
![]() eCTD structure fabrication
eCTD structure fabrication
According to the specific information of the Tracking Log, use the eCTD publisher to upload the edited files from M1 to M5 to establish an eCTD framework that meets the eCTD requirements. Make cross-file links in the eCTD file structure, perform QC and verification, and check link properties and file properties in batches;
-
![]() eCTD Verification
eCTD Verification
Validate a complete set of eCTD application materials according to the Chinese eCTD verification standard, generate a verification report with detailed content and clear structure, which can accurately locate relevant documents or content, and solve and repair the warning and error prompts in the verification report until the standard verification is correct and conforms to the eCTD structure specification. electronic submission of documents.

Word file check
PDF file creation
eCTD structure fabrication
eCTD Verification